From laboratory to clinic: AMBLor® melanoma prognostic is now available to healthcare professionals
In this article
AMBLor: A groundbreaking test for early-stage melanoma prognosis
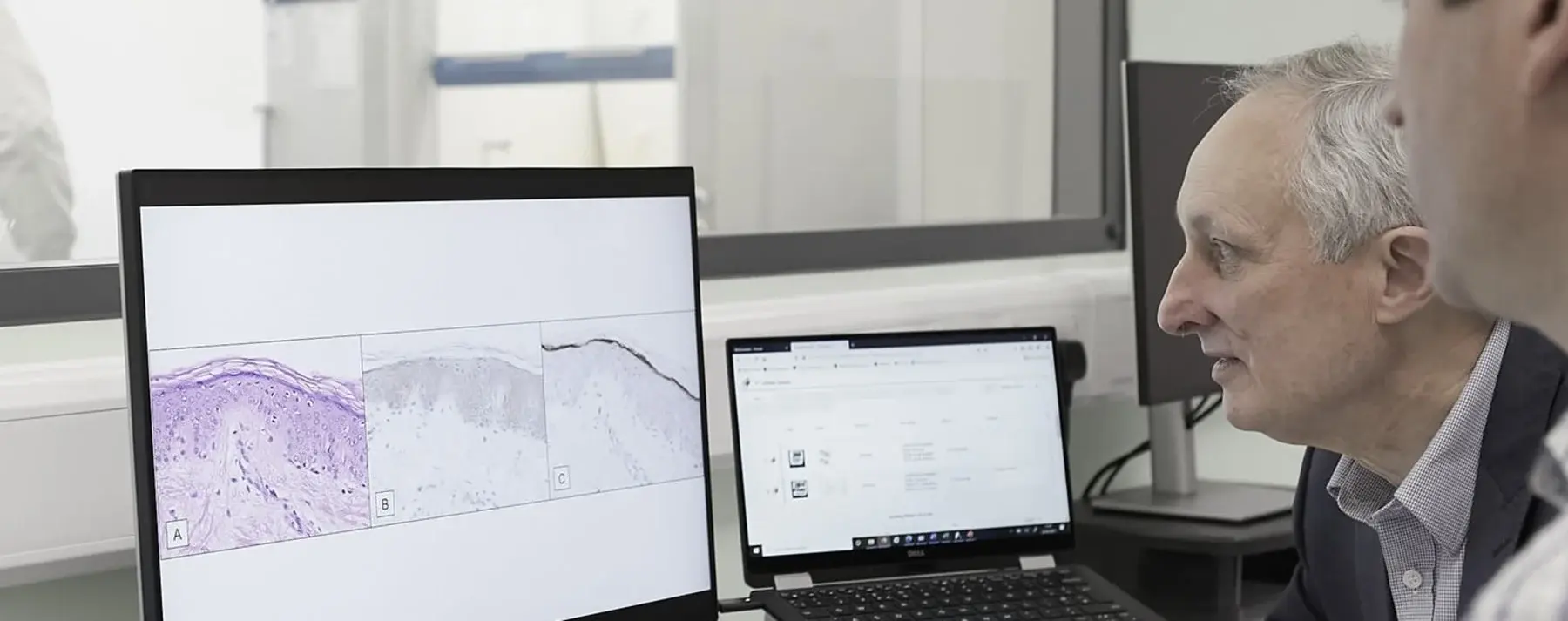
Early innovative research that was partly funded by the British Skin Foundation has now been developed into a prognostic test that received its UKCA mark this month, meaning it is available to hospitals and clinics across the country. AMBLor is a groundbreaking, histopathological biomarker test from AMLo Biosciences that enables accurate, personal identification of early-stage melanomas that are at low risk of progression.

British Skin Foundation’s support in early research
The British Skin Foundation supported this research at laboratory stage with Professor Penny Lovat of Newcastle University investigating two protein biomarkers named AMBRA1 and loricrin. These biomarkers are present in the epidermis layer of normal healthy skin and the research discovered that the loss of these markers in patients with early-stage melanoma is associated with tumours at-risk of spreading, whereas if the markers are retained there is a significantly reduced risk of their tumour spreading.
Clinical studies and development of AMBLor
Following a number of clinical studies and published papers demonstrating high levels of accurate prediction, these biomarkers now comprise AMBLor, the test that can be added to the existing early-stage melanoma diagnostic laboratory tests to provide additional understanding of a patient’s prognosis.

Addressing the lack of prognostic information
Only around 20% of early stage I or II melanomas actually progress to metastatic disease, and until now there has been no accurate way to predict if a melanoma will reoccur. As a result of this lack of prognostic information, all patients have had to follow the same disease management guidance, sometimes requiring invasive sentinel lymph node biopsy, other imaging tests and up to five-years of follow-up appointments and scans. Patients often experience anxiety around appointments that can be so severe it affects daily life.
Potential to reduce strain on healthcare systems
With NHS waiting lists for skin cancer ever increasing, the use of AMBLor in everyday clinical practice could segment a group of patients at low risk of melanoma spread that could be spared further diagnostic procedures and lengthy follow-up schedules. This would alleviate the pressure on clinical teams and resources.
Celebrating the AMBLor milestone
We are thrilled that one of our funded projects has achieved this remarkable milestone, now offering tangible benefits to melanoma patients. This success highlights the dedication of AMLo Biosciences and the critical role of innovative research in advancing skin disease management. We look forward to supporting many more early-stage research projects in achieving their goals.
Lisa Bickerstaffe, Head of Communications at the British Skin Foundation
Looking ahead
The team at AMLo are thrilled to receive the UKCA mark for AMBLor. We want to thank the British Skin Foundation for the initial funding and the ongoing support throughout our development journey. We are excited to follow the positive impact our prognostic can have on melanoma patients and healthcare professionals.
Dr Marie Labus, CEO AMLo Biosciences
Donate to us now
By donating to skin disease research, you are helping us to find treatments and cures for common conditions like rosacea, acne and psoriasis through to potential killers like melanoma skin cancer. Thank you.